Lihat Semua : infografis
EUA Indovac, Vaksin Pertama Buatan Indonesia TERBIT
Dipublikasikan pada 2 years ago , Redaktur: Andrean W. Finaka, Riset : Yuli Nurhanisah / Desain : Chyntia Devina / View : 4.410 |
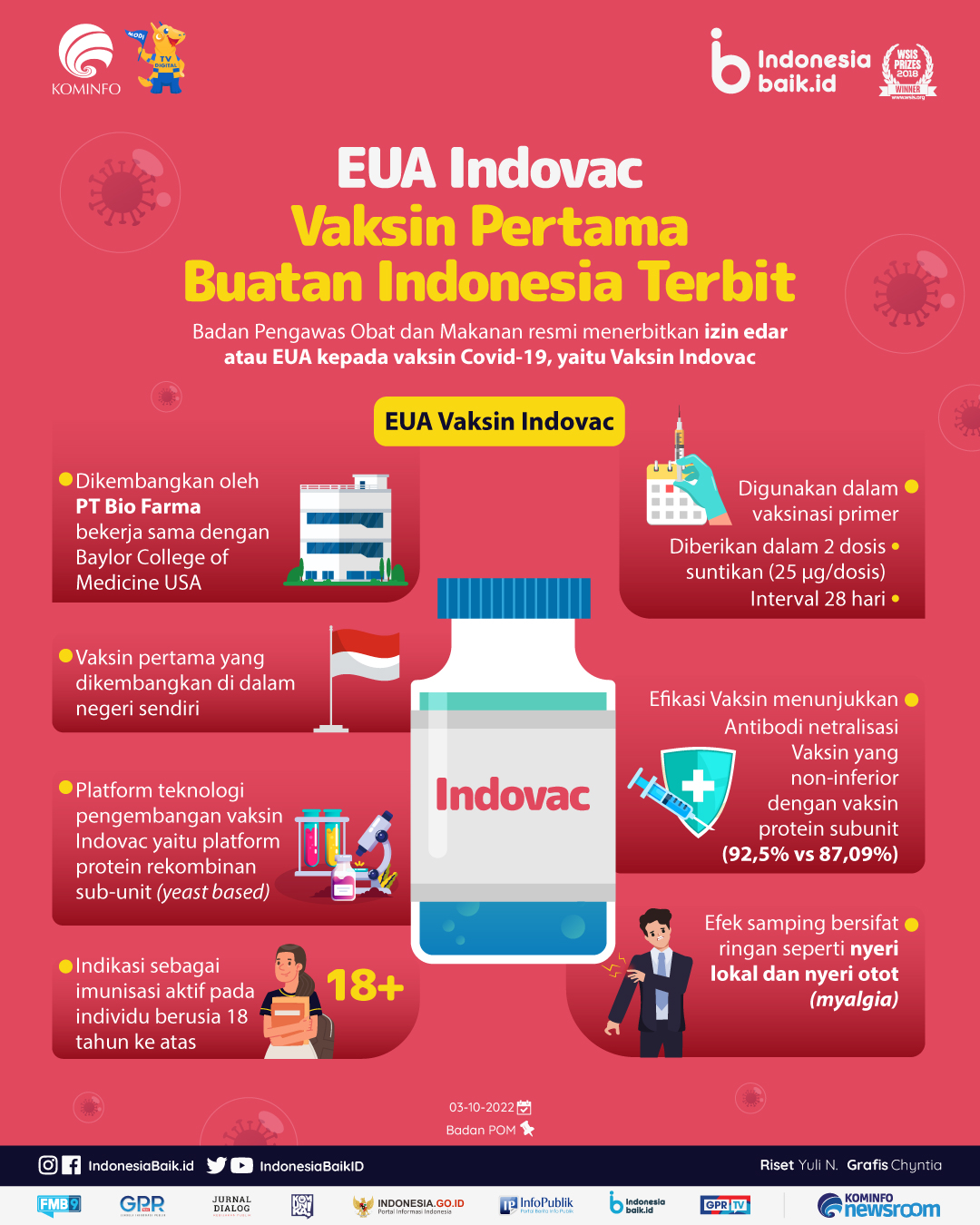
Indonesiabaik.id - Badan Pengawas Obat dan Makanan (BPOM) resmi menerbitkan izin edar atau Emergency Used Authorization/EUA kepada vaksin Covid-19, yaitu Vaksin Indovac.
EUA Vaksin Indovac
Vaksin Indovac dikembangkan oleh PT Bio Farma bekerja sama dengan Baylor College of Medicine USA. Vaksin Indovac adalah vaksin pertama yang dikembangkan di dalam negeri sendiri. Platform teknologi pengembangan vaksin Indovac yaitu platform protein rekombinan sub-unit (yeast based).
EUA Vaksin Indovac dengan indikasi sebagai imunisasi aktif untuk pencegahan COVID-19 yang disebabkan oleh SARS CoV-2 pada individu berusia 18 tahun ke atas.
Vaksin Indovac akan digunakan dalam vaksinasi primer yang diberikan dalam 2 dosis suntikan (25 μg/dosis) dengan interval 28 hari. Efikasi Vaksin Indovac mengacu pada hasil uji imuno bridging pada uji klinik fase 3, menunjukkan antibodi netralisasi Vaksin yang non-inferior dengan vaksin protein subunit pembanding (92,5% vs 87,09%).
Efek samping atau adverse events (AEs) dalam uji klinik Vaksin Indovac dilaporkan umumnya bersifat ringan. Efek samping yang paling sering dilaporkan adalah nyeri lokal dan nyeri otot (myalgia).
=================================================
EUA Indovac, the First COVID Vaccine Made in Indonesia is Released
Indonesiabaik.id - The COVID-19 vaccine Indovac has received an Emergency Used Authorization (EUA) from the Food and Drug Supervisory Agency (BPOM).
Indovac Vaccine EUA
The Indovac vaccine was developed by PT Bio Farma in collaboration with Baylor College of Medicine USA. The Indovac vaccine is the first vaccine developed domestically. A sub-unit (yeast-based) recombinant protein platform serves as the platform for Indovac's vaccine development technology.
EUA Indovac vaccine is indicated as an active immunization for the prevention of COVID-19 caused by SARS-CoV-2 in those 18 years of age and above.
Indovac vaccine will be used in primary vaccination which is administered in 2 injection doses (25 μg/dose) with an interval of 28 days. Indovac Vaccine Efficacy refers to the results of the immune-bridging test in phase 3 clinical trials, showing vaccine neutralizing antibodies that are non-inferior with the comparator protein subunit vaccine (92.5% vs 87.09%).
The Indovac Vaccine clinical trial found that adverse events (AEs) were mostly minor. Local and muscle soreness (myalgia) are the side effects that are most frequently reported.